EU Guide: Basic UDI-DI
January 3, 2022
UK updates post-Brexit medical device guidance
January 4, 2022UK Steps to Market
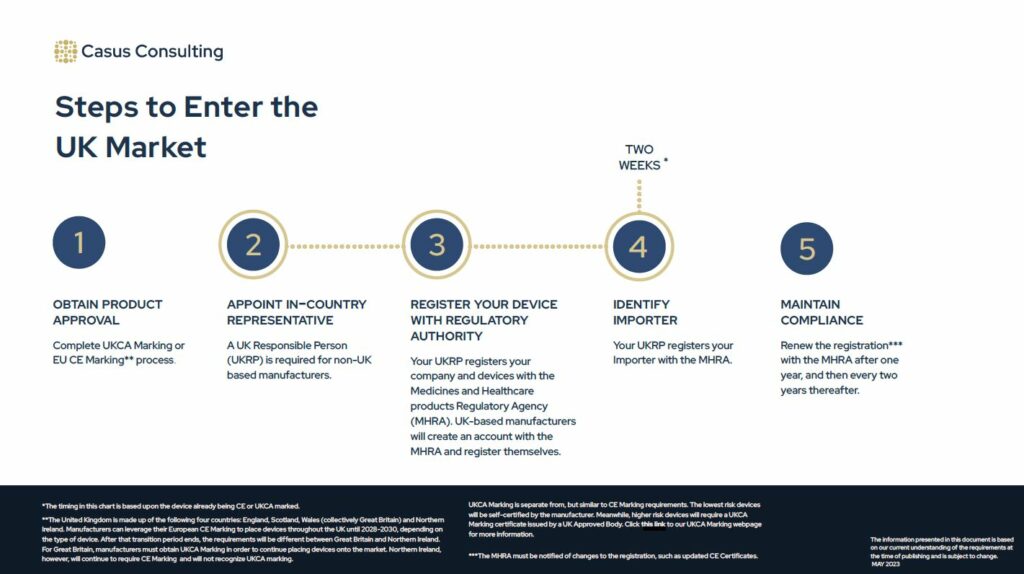
Steps to Enter the UK Market
Estimated Timeline: 2 Weeks, if the device is already CE Marked or UKCA Marked. If the device is not CE or UKCA Marked, the process may take an additional 2-4 months (lowest risk device) to 1-2 years (mid-to-high risk device).
1. Obtain product approval
-
- Complete EU CE Marking or UK Conformity Assessment (UKCA Marking).
- Manufacturers can leverage their European CE Marking to place devices throughout the UK until 2028-2030, depending on the type of device.
- BY 2030, UKCA Marking becomes mandatory for Great Britain. For more information on the deadlines, read our page: UK MHRA Annoucement: New Dates for UK Regulation & CE Marking
- IMPORTANT: the requirements will be different between Great Britain and Northern Ireland. For Great Britain, medical device manufacturers must obtain UKCA Marking by 2028-2030 (depending on the type of device), in order to continue placing devices onto the market. Northern Ireland, however, will continue to require CE Marking after this time.
- UKCA Marking is separate from, but similar to CE Marking requirements. The lowest risk devices will be self-certified by the manufacturer. Meanwhile, higher risk devices will require a UKCA Marking certificate issued by a UK Approved Body.
2. Appoint in-country representative (UK Responsible Person)
-
- A UK Responsible Person (UKRP) is required for non-UK based manufacturers.
- The MHRA granted transition deadlines over the course of 2021 for manufacturers to appoint a UKRP. However, the transition period is now over. As of 1 January 2022, a UKRP is mandatory to place medical devices onto the market.
- The UKRP’s role is currently similar to the role of the EU Authorized Representative (AR) under the old European Directives. However, the MHRA has proposed changes to the role within their public consultation published in September 2021. These changes increase the responsibilities and liability of the UKRP, making the role more similar to the EU AR under the new European Medical Device & IVD Regulations.
- For more information, read our page: UK Responsible Person
3. Register your device with MHRA
-
- Your UKRP registers your company and devices with the Medicines and Healthcare products Regulatory Agency (MHRA). UK-based manufacturers will create an account with the MHRA and register themselves.
- Basic information about the device and manufacturer are submitted as part of the registration process, along with the manufacturer’s declaration of conformity (for self-certified products) or conformity assessment certificate.
- As part of the registration process, the UKRP must provide documentation proving that you have granted authority for the company to act as the UKRP, such as a Letter of Designation or copy of the UKRP Agreement.
- The MHRA quotes up to 5 days to process the application. The information in the application and timing are generally the same for all risk classes of devices.
- Once the device is registered, it will appear in the MHRA’s public database. The database is searchable by manufacturer and GMDN code.
- For more information on information needed in the registration, timing and fees, visit our page: UK MHRA Medical Device & IVD Registration
4. Register Importer (if applicable)
-
- Your UKRP must register your importer(s) with the MHRA. The importer information does not appear in the MHRA’s public database.
- If you sell direct (e.g., direct download of a standalone software device), then this step may not be needed.
- Distributors and other suppliers are not required to be registered with the MHRA.
5. Maintain compliance
-
- Maintain CE Marking / UKCA Marking.
- Renew the registration with the MHRA after one year, and then every two years thereafter.
- The MHRA must be notified of changes to the registration, e.g., updated CE/UKCA Certificates, changes to the manufacturer’s address, etc.