MDR CE Marking Overview
In order to sell your medical devices in Europe, they must first be CE Marked. CE Marking is Europe’s version of marketing approval.
Prior to CE Marking a medical device, manufacturers must compile technical and clinical documentation to demonstrate compliance with the EU Medical Device Regulation (MDR) 2017/745.
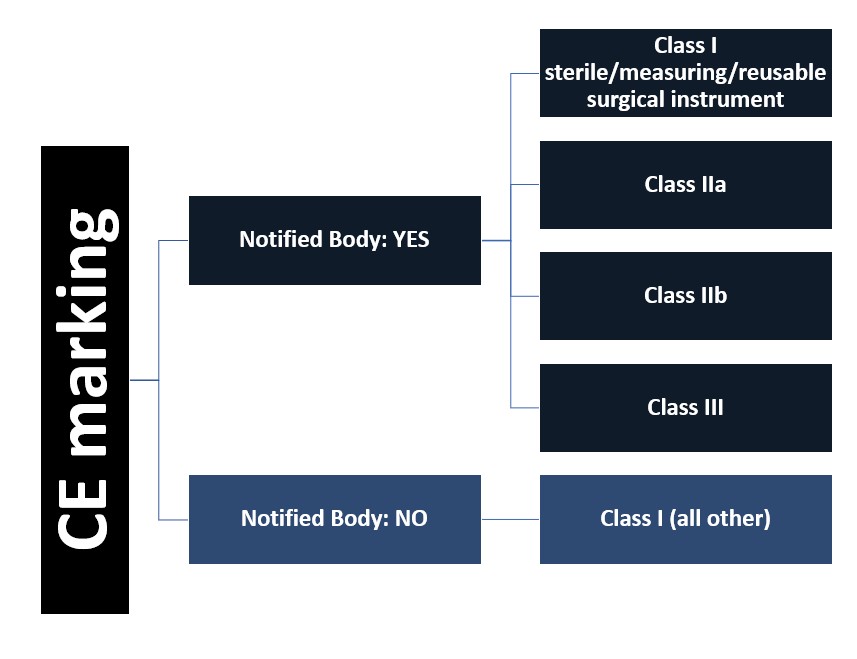
Further, unless the device is the very lowest risk class, it must undergo a conformity assessment review by a Notified Body. Notified Bodies are organizations accredited and supervised by the European National Competent Authorities, and their role is to evaluate if products have met the minimum safety and efficacy requirements. The Notified Body issues “CE Mark Approval” for the device by issuing a CE certificate.
If the device is the lowest risk, then it does not require a CE certificate issued by a Notified Body. Instead, the manufacturer must compile the safety and efficacy information, and then they will “self-certify” the device. They will affix the CE symbol on their product and sign a Declaration of Conformity, which is a legal document, asserting that they have met the minimum requirements of the regulation.
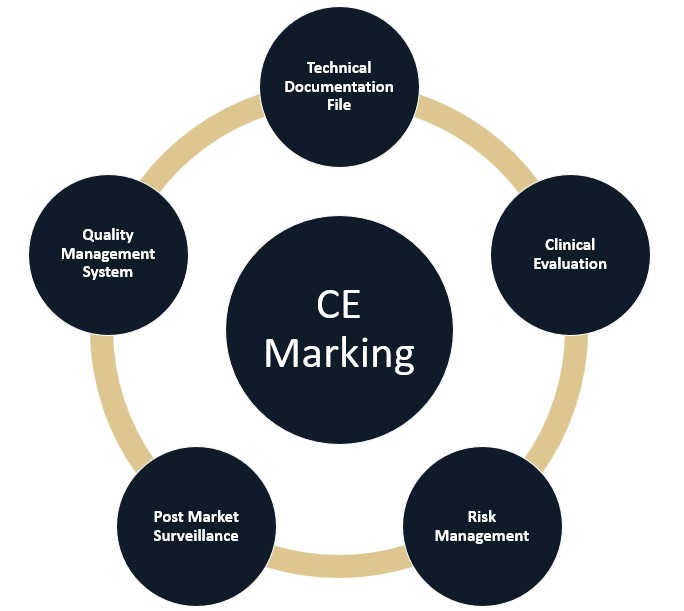
Below we have broken down each element of the technical file documentation. Casus takes a practical, business minded approach to assisting manufacturers achieve CE marking. We are available to work with you on a single element of your documentation or full support.
Technical Documentation File Overview
All medical devices, irrespective of classification and risk level, require the following elements:
-
- MDR Annex II – Technical Documentation File (TDF), which includes information related to the product design and manufacturing, product verification and validation, and risk analysis and management
- MDR, Annex III – Post-Market Surveillance (PMS) Plan
- MDR, Annex XIV – Clinical Evaluation Report (CER)
Not all the elements outlined within each section above may apply to your device. Manufacturers should determine which sections within each Annex (TDF/PMS/CER) are applicable for their device. Where a section is deemed not applicable, manufacturers should document a rationale as to why this has been excluded from the Technical Documentation File (TDF) and Clinical Evaluation Report (CER).
The information detailed in the TDF/CER should provide evidence that the General Safety and Performance Requirements (GSPRs), described in more detail below, have been sufficiently addressed.
Casus Consulting offers compilation services for both the TDF and CER. We can help you understand how the MDR applies to your device and the required information to be included in each file. We can also provide support in conducting a gap analysis of your ‘legacy’ device technical files and upgrading them to the MDR.
MDR Key Elements
Manufacturers should consider the technical file as a whole when drafting, rather than looking at the sections as standalone items. For example, the General Safety and Performance Requirements (GSPRs) include information from the Risk Management section, and elements of the Risk Management section need to be considered for the Post-market Surveillance plan.
1) Technical Documentation File Main Body (MDR Annex II)
The main body of an MDR compliant Technical Documentation File (TDF) should include key details and documentation related to the device, including the description, labeling/instructions for use, design and manufacturing information, and product verification and validation (i.e., data to prove the safety and efficacy of the device).
Two key components that manufacturers need to assess for the TDF are the General Safety and Performance Requirements and Risk Management section.
GSPRs – General Safety and Performance Requirements
-
- The GSPRs detail the specific criteria manufacturers must meet to establish minimum safety and efficacy requirements for their device, e.g., requirements for performance, risk management, design, etc.
- The GSPRs, located in Annex I of the MDR, are similar to the Essential Requirements Checklist (ERC), located in Annex I of the old Medical Devices Directive (MDD). Manufacturers with MDD CE Marked devices can generally leverage aspects of their ERC to draft the GSPRs. However, there will be several gaps to address.
- European Harmonized Standards should be utilized, where available. The reason is that use of harmonized standards ‘presumes compliance’ to the applicable requirement.
Risk Analysis & Management
-
- Manufacturers must assess possible risks related to their device and conclude that there is a positive risk-benefit ratio in order to CE Mark the device. This is done in various ways, including rating the probability of occurrence against the severity of harm if it does occur. Manufacturers should detail the procedures in place to mitigate the risk or actions to take if it occurs.
- EN ISO 14971:2019, for medical device risk management, is the harmonized standard under the EU MDR.
- Annex I, section 3 of the MDR includes specific criteria that manufacturers must meet when implementing their risk management system. This section states that risk management is “a continuous iterative process throughout the entire lifecycle of a device, requiring regular systematic updating”.
2) Post-Market Surveillance (MDR Annex III)
Post-market Surveillance (PMS) Plan
-
- The Post Market Surveillance (PMS) section of the TDF details the manufacturer’s plan to collect and assess post-market data. The PMS Plan should include information from serious incidents, complaints, public information on other similar medical devices, and more (refer to MDR Annex III, Section 1(a)).
- The PMS Plan must also establish how the manufacturer will use the data collected to take action when needed. Actions may include making updates to device documentation, such as the CER, improving the risk management and/or taking preventative or corrective action.
Post-market Clinical Follow-up (PMCF) Plan
-
- The Post-market Clinical Follow-up (PMCF) plan is a subset of your PMS Plan and is very much intertwined with your risk management and clinical evaluation. The PMCF plan is intended to help identify previously unknown risks and to confirm the device’s safety and performance.
- A PMCF Plan can include a PMCF study when warranted. However, other methods can also be used to address gaps in your pre-clinical data and to demonstrate a continued positive benefit-risk ratio, when appropriate. Examples include feedback via patient or physician surveys and screening of scientific literature.
Post-Market Surveillance Report (PMSR)
-
- Manufacturers of Class I devices must prepare a post-market surveillance report (PMSR) based on data collected once the product is on the market. The PMSR is generated through review and analysis of the gathered PMS data and should also include any preventative or corrective actions taken.
- The MDR states the PMSR should be updated ‘when necessary’.
Periodic Safety Update Report (PSUR)
-
- Manufacturers of Class IIa, IIb and III devices are required to prepare a Periodic Safety Update Report (PSUR) for each device.
- The PSUR is a living record in which the manufacturer systematically assesses their PMS data to ensure the device continues to be safe and effective and to provide an accurate picture of the products’ performance post-market. This includes any preventative or corrective actions taken and volume of sales.
- Manufacturers of Class IIa devices must update the PSUR at least every two years. Manufacturers of Class IIb and III devices must update their PSUR at least annually. Further, manufacturers of implantable or Class III devices must provide the PSUR to their notified bodies and it will be accessible to all national competent authorities and the EU Commission.
3) Clinical Evaluation Report (MDR Annex XIV)
The Clinical Evaluation Report (CER) is a crucial element of the technical file, and includes the following information:
-
- basic device information,
- Clinical Evaluation Plan (CEP),
- common specifications and harmonized standards,
- a clinical literature review,
- data from a clinical investigation (if required),
- Post-Market Surveillance (PMS) data,
- Post-Market Clinical Follow-Up (PMCF) data,
- a plan for continual updates to this section, and
- other relevant device documentation, such as the IFU.
The CER is a living document that should be continually updated: “clinical evaluation and its documentation shall be updated throughout the life cycle of the device concerned with clinical data obtained from the implementation of the manufacturer’s PMCF plan … and the post-market surveillance plan...”.
Other elements of CE Marking
Manufacturers are expected to have a minimum quality management system in place, including the lowest risk Class I device manufacturers. Further, manufacturers should have a system for incident reporting and managing field safety corrective actions (FSCAs), such as device recalls.