February 21, 2022
Manufacturers selling in the EU, UK and Switzerland need to consider the different labeling requirements for listing their in-country representatives ...
February 18, 2022
Is it possible to transfer your UKRP? Can I appoint more than one UKRP? Is a Transfer Agreement needed between ...
January 31, 2022
Need a GMDN code? Find here the three best ways to find GMDN codes for free. Plus, how to confirm that you selected the correct code & other GMDN FAQs.
January 18, 2022
Below is an overview of the Swiss Single Registration Number and how it applies to you. What is a Swiss ...
January 5, 2022
In short, legacy devices are: MDD 93/42/EEC Class I self-certified devices, which have been up-classed under the MDR and therefore ...
January 3, 2022
While Basic UDI-DI was created to help streamline device identification, it continues to be a confusing concept to many.
January 3, 2022
Do you need a European Free Sale Certificate for export, but not sure how to get one? Find out here who is allowed to request one, the cost and time.
October 10, 2021
EUDAMED Module: UDI / Device Registration Once your Single Registration Number has been issued, you are ready to move foward ...
October 10, 2021
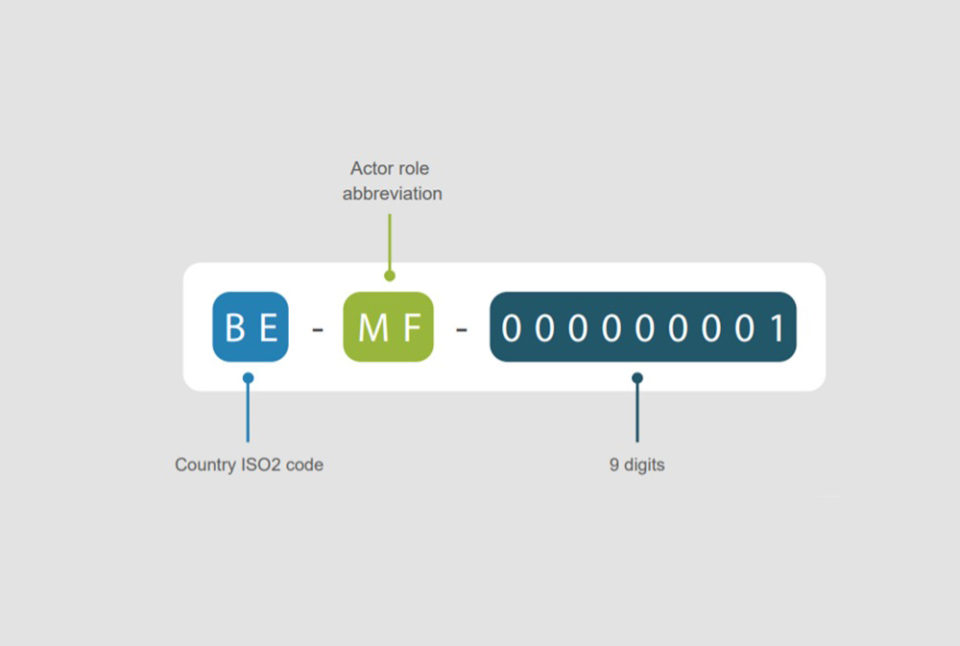
EUDAMED Module: Actor Registration Company registrations are submitted in the EUDAMED Actor Registration Module. What is an Actor? Per the ...