EU Steps to Market
January 6, 2022
What is a CHRN (Swiss Single Registration Number)?
January 18, 2022Switzerland Steps to Market
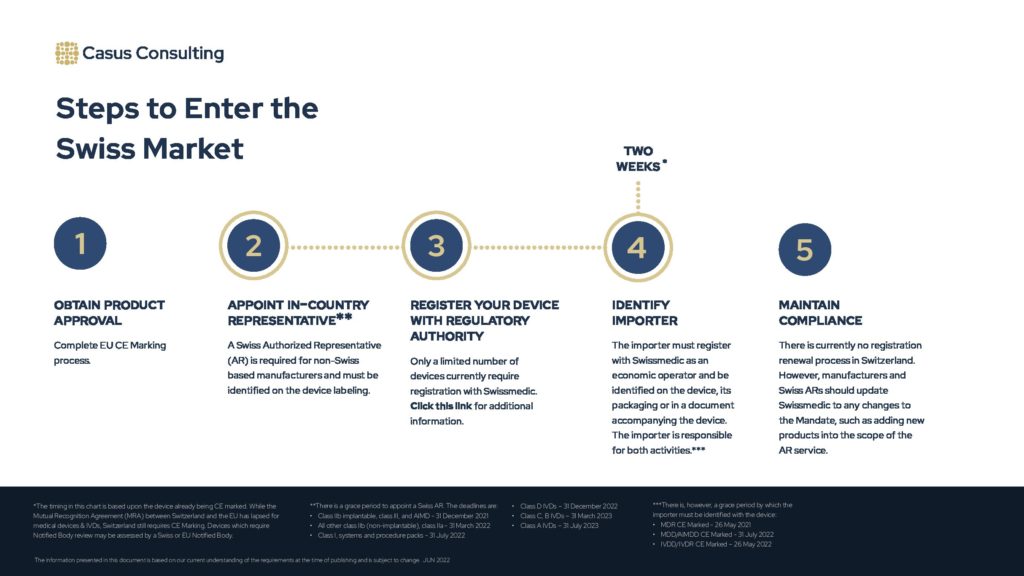
The above chart provides a high-level overview of the steps to place medical devices and IVDs onto the market in Switzerland.
The overall process is also detailed below and here: How to sell your medical devices and IVDs in Switzerland
Steps to Enter the Swiss Market
Estimated Timeline: 2 weeks, if the device is already CE marked.
Obtain product approval
-
- Complete European CE Marking process. Lowest risk devices are self-certified by the manufacturer, while higher risk devices must obtain CE Marking certification through a Notified Body.
- While the Mutual Recognition Agreement (MRA) between Switzerland and the EU has lapsed for medical devices and IVDs, Switzerland still requires CE Marking. Swissmedic accepts CE Marking certificates issued by both Swiss and EU Notified Bodies.
- For more information on the CE Marking process, visit: MDR CE Marking, IVDR CE Marking
Appoint in-country representative
-
- A Swiss Authorized Representative (aka CH-REP) is required for all manufacturers located outside of Switzerland*.
- There is a grace period to appoint a CH-REP:
- Medical devices: Class III/AIMD/Class IIb implantable – 31 December 2021; Class IIb non-implantable/Class IIa – 31 March 2022; Class I – 31 July 2022; Systems/Procedure Packs – 31 July 2022
- IVDs: Class D – 31 December 2022; Class C/B – 31 March 2023; Class A – 31 July 2023
- For more information, please visit: Switzerland Authorized Representative
*Due to a customs treaty between Switzerland and Liechtenstein, a manufacturer located in Liechtenstein is not required to appoint a Swiss Authorized Representative.
Register your device with regulatory authority
-
- Only a limited number of medical devices require registration at this time: Class I devices, Systems/Procedure Packs, custom-made devices and IVDs.
- NOTE: only Swiss-based manufacturers are required to register their Class I devices, Systems/Procedure Packs and IVDs with Swissmedic. Swiss Authorized Representatives (AR) are not required to register these devices on behalf of the clients they represent. However, Swiss ARs are required to register custom-made devices on behalf of their non-Swiss clients.
- Swiss-based manufacturers and authorized representatives must register with Swissmedic and obtain a Swiss Single Registration Number ( CHRN ). Unlike in Europe, where the Single Registration Number is voluntary until EUDAMED is fully functional , the CHRN is mandatory. If you are a manufacturer located outside of Switzerland, you do not require a CHRN.
- For more information, please visit: Switzerland Registration
- Only a limited number of medical devices require registration at this time: Class I devices, Systems/Procedure Packs, custom-made devices and IVDs.
Identify Importer
-
- The Swiss Medical Device Ordinance (MedDO/IvDO) defines an importer as ’any natural or legal person established within Switzerland that places a device from a foreign country on the Swiss market’.
- Importers must register with Swissmedic and obtain a CHRN .
- Importers must identify themselves on the device label, packaging, or in a document accompanying the device. NOTE: Swissmedic has a broader definition of ‘a document accompanying the device’ than Europe. Swissmedic allows the information to be presented in information such as the delivery note or invoice, and further states that, unlike in Europe, ‘the “document accompanying the device” does not necessarily need to reach the end user.’
- The importer must be identified with the device by the following:
- MDR CE Marked – 26 May 2021; MDD/AIMDD CE Marked – 31 July 2022
- IVDD/IVDR – no grace period; must be identified as of 26 May 2022
- Distributors are not required to register with Swissmedic, do not require a CHRN and are not required to identify themselves on the device label, packaging or document accompanying the device.
Maintain Compliance
-
- Maintain CE Marking. This includes transitioning compliance from the old Directives to the new Regulations, as applicable.