EU Guide: What are Legacy Devices?
January 5, 2022
Switzerland Steps to Market
January 15, 2022EU Steps to Market
The above chart provides a high-level overview of the steps to place medical devices and IVDs onto the European Single Market . The overall process is also detailed below.
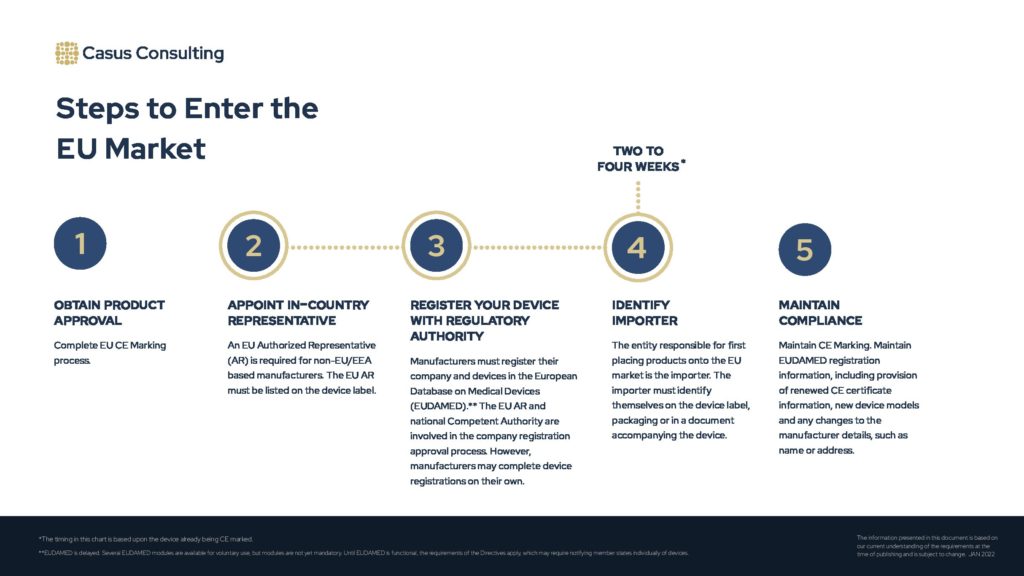
Steps to Enter the European Market
Estimated Timeline: 2-4 weeks, if the device is already CE marked.
Obtain product approval
-
- Complete European CE Marking process. Lowest risk devices are self-certified by the manufacturer, while higher risk devices must obtain CE Marking certification through a Notified Body.
- For more information on the process, please read: MDR CE Marking, IVDR CE Marking
Appoint in-country representative
-
- A European Authorized Representative (aka EC-REP) is required for manufacturers not located in the Great Britain and Switzerland , who now require an EU Authorized Representative.
- For more information, please read: European Authorized Representative
Register your device with regulatory authority
-
- Manufacturers must register their company and devices in the European Database on Medical Devices (EUDAMED).
- The manufacturer registration is completed within the EUDAMED ‘Actor Registration’ Module. The EU Authorized Representative and national Competent Authorities are involved in the manufacturer registration approval process. After the registration has been approved, a European Single Registration Number (SRN) will be issued.
- The medical device registration is completed within the EUDAMED ‘UDI/Device Registration’ Module. For the most part, manufacturers complete device registrations on their own. The EU Authorized Representative and national Competent Authorities are not involved, although in certain cases a Notified Body may need to review the registration.
- IMPORTANT: Until EUDAMED is fully functional, use is voluntary. Therefore, the above process will only be required once EUDAMED is mandatory. In the meantime, registration requirements under the old Directives continue to apply, which may require notifying member states individually of devices.
- For more information on the process and timing, please read: What is an SRN?, EUDAMED Basics, EUDAMED Actor Registration, EUDAMED Device Registration
Identify importer
-
- The MDR/IVDR now defines the role and responsibilities of the importer. The importer is ‘any natural or legal person established within the Union that places a device from a third country on the Union market’.
- Importers must register in the EUDAMED Actor Registration module and obtain a Single Registration Number (SRN). They must then link themselves with the manufacturer and devices they import. NOTE: Until EUDAMED is fully functional, use is voluntary. Therefore, the above process will only be required once EUDAMED is mandatory.
- Importers must identify themselves on the device label, packaging or in a document accompanying the device. Their information must accompany the device through the supply chain and reach the end user.
- Distributors are not required to register in EUDAMED, nor are they required to identify themselves on the device label, packaging or document accompanying the device.
Maintain compliance
-
- Maintain CE Marking. This includes transitioning from the old Directives to the new Regulations, as applicable.
- If you have not voluntarily registered in EUDAMED, do so when EUDAMED registration becomes mandatory. Currently EUDAMED is expected to be fully functional Q2 2024, followed by a transition period.
- Update EUDAMED registration when any changes occur, such as addition of new device models, updates to CE marking certificates and changes to manufacturer name or address.