‘Placing on the Market’: Definition and Cutoff Dates for EU, UK & Switzerland

MHRA Updates List of UKCA Approved Bodies
May 17, 2022
New Standards Harmonized to MDR/IVDR, including ISO 14971
May 19, 2022Page Last Reviewed: 2 January 2024
Europe is in a state of regulatory flux. There are numerous cutoff dates within the next five years. As a result, manufacturers must know by which date they can no longer place stock that do not comply with the new regulations (e.g., UKCA, IVDR) onto the market.
General criteria for products to be considered ‘placed on the market’
Below is a high-level overview and a set of examples, after which manufacturers should not place any old regulation devices onto the market.
| Market | Products must be ready for distribution (i.e., must already be manufactured) | Transfer of Ownership must be complete | Deadline for legacy devices to be ‘placed on the market’ |
| EU | YES | YES* | Varies by market, regulation, and classification. |
| UK | YES | YES* | |
| CH | YES | YES** |
*For the EU & UK, transfer of ownership can be an agreement to purchase executed between manufacturer & local importer/customer.
**For Switzerland (CH), the transfer of ownership for a non-Swiss manufacturer’s products is between the Swiss Importer and the Swiss customer, and not between the foreign manufacturer and the Swiss Importer. See Switzerland section below for more details.
Europe: definition of ‘placing on the market’
The Blue Guide defines ‘placing on the market’ as:
“A product is placed on the market when it is made available for the first time on the Union market. This operation should be done by the manufacturer or by an importer.
When a manufacturer or an importer supplies a product to a distributor or an end-user for the first time, the operation is always labelled in legal terms as ‘placing on the market’. Any subsequent operation, for instance, from a distributor to distributor or from a distributor to an end-user is defined as making available.“
It further states:
“Placing a product on the market requires an offer or an agreement (written or verbal) between two or more legal or natural persons for the transfer of ownership, possession or any other property right concerning the product in question; it requires that the manufacturing stage has been completed.
This transfer could be for payment or free of charge. It does not require the physical handover of the product.
Sometimes products are manufactured following the placing of an order. An offer or agreement concluded before the stage of manufacture has been finalised cannot be considered as placing on the market (e.g. an offer to manufacture a product according to certain specifications agreed by the parties to the contract, where the product will only be manufactured and delivered at a later stage).“
What does this mean in practical terms?
The key points are:
- ‘requires that the manufacturing stage has been completed’
- ‘transfer of ownership’
- ‘does not require physical handover of the product’
Based on this, a manufacturer can have finished products warehoused and ready for use. They can then transfer the ownership of the products to an EU customer, e.g., agreement to sell the product to an EU importer. That date of sale (‘transfer of ownership’) is the date the device was placed onto the market. The devices can then be shipped at a later date.
The EU Commission’s “Notice to Stakeholders: Withdrawal of the United Kingdom and EU Rules in the Field of Industrial Products” reiterates the Blue Guide. While this Notice was drafted specifically regarding ‘Brexit’, the information can be applied to other situations in which ‘placing on the market’ is key, including legislation cutoff dates.

MDR/IVDR Importer Requirements & FAQs
Importer requirements under Article 13 of the MDR/IVDR. Plus, what are the manufacturer’s obligations to/from the importer and how are direct sales handled?
Europe: Example Scenarios
Per the EU Commission’s Notice to Stakeholders:
Situations which are considered as ‘placing on the market’ include:
- Contract of sale from manufacturer to importer, distributor (also intra-group provided a genuine transaction can be identified) or final customer, where the manufacturing of that good has been completed;
- On-line sales: only when the customer receives confirmation of his order which identifies the specific good already manufactured and subject of the transaction, ready to be shipped to the customer
Situations that are not considered as ‘placing on the market’:
- Pre-ordered goods, not yet manufactured
- Contract for the supply of fungible goods (e.g. x units of product y, not individually identifiable)
- Generic offer of a product on-line (i.e., they are placed on the market at the moment an order by an end user has been placed and confirmed for a specific product already manufactured and subject of the transaction, and ready to be shipped.)
Example Situation: IVDR date of application
You manufacture IVDs. You have an IVDD CE marked device, that must comply with the IVDR on its date of application: 26 May 2022. The devices are already manufactured, under IVDD CE marking, and ready to be shipped. An EU importer has agreed to purchase the products on 25 May 2022. The products will not physically enter Europe until June 2022.
In the above scenario, the IVDD CE marked products were placed on the market 25 May 2022, before the IVDR’s date of application. Therefore, they can be shipped to your customer at a later date, since the products are already considered placed on the market.
It is the Economic Operators’ responsibility to bear:
“the burden of proof of demonstrating on the basis of any relevant document that the good was placed on the market…Such proof can be given on the basis of documents ordinarily used in business transactions (e.g. contract of sale concerning goods which have already been manufactured, invoice, documents concerning the shipping of goods to distribution or similar commercial documents). There is no need to create a new type of document for this purpose.“
Are my devices considered ‘placed on the market’ if transferred to an EU third party logistics (3PL) provider?
The question here is, are you transferring ownership of the goods, as defined by the guidance documents? Or only shipping the goods for the 3PL to warehouse, for later transfer of ownership?
If the 3PL contractually is not the importer and does not take legal ownership of the devices, then the products are not considered placed onto the market at that time. Instead, the devices will be considered placed on the market after you direct products to be transferred to (or on behalf of) your European customer. That customer will then assume the role of the EU importer (even though the products are already in Europe, at the 3PL’s warehouse), and the products are considered placed onto the market at that time.
For more information on this topic, please refer to: MDR/IVDR Guidance for Importers and Distributors
UK: definition of ‘placing on market’
The United Kingdom (UK) left the European Union (EU) in 2020. As a result, the UK has transitioned to its own separate regulatory system. However, the UK currently has the same definition of ‘placing on the market’ as the EU.
The definition can be found on the MHRA’s website: Placing Manufactured Goods on the Market in Great Britain
“A fully manufactured good is ‘placed on the market’ when a written or verbal agreement (or offer of an agreement) to transfer ownership or possession or other property rights in the product is exchanged.
‘Placing a good on the market’ means each individual good, not a type of good. It does not require the physical transfer of the good.
You can usually provide proof of placing on the market on the basis of any relevant document ordinarily used in business transactions, including:
- contracts of sale concerning goods which have already been manufactured and meet the legal requirements
- invoices
- documents concerning the shipping of goods for distribution
The relevant economic operator (whether manufacturer, importer or distributor) bears the burden of proof for demonstrating that the good was placed on the market…”
The MHRA also issued an unambiguously titled document: Notice – Definition of ‘placing on the market’ before and after the UK leaves the EU, if there’s no Brexit deal
As with the EU Commission’s document related to ‘Brexit’ , the information in the MHRA’s Notice can be taken to apply to other scenarios related to ‘placing on the market’.

UKCA Marking Fact Sheet
UKCA Marking deadlines; UKCA versus CE Marking technical file and labeling differences; where to download the symbol; and more.
UK: Example Scenarios
Samples from the Notice document are provided below. These situations can also be applied to mean: non-UK manufacturer placing devices onto the Great Britain market.
Scenario 1: Product manufactured by an EU-27 company and shipped to their UK subsidiary’s warehouse before the point of exit in a ‘no deal scenario’. The subsidiary then distributes the product to customers in the UK after the withdrawal date.
The product will be considered to be placed on the UK market when there is an agreement to supply it on the UK market for the first time. Therefore, in this instance, when the product is placed on the UK market will depend when the offer or agreement to supply takes place.
If there is an offer or agreement for the transfer of ownership or possession (or other right in the product) between the manufacturer and its UK subsidiary, then the date the product is placed on the market will be the date of that transaction.
Scenario 2: Products manufactured in the UK, or imported into the UK from a third country, that are offered for sale online to customers based in the EU-27 by sellers based in the UK after the point of exit in a no deal scenario.
Note: Since both markets define ‘placing on the market’ the same way, the example scenarios from the Europe section can also be applied for the UK.
Switzerland: definition of ‘placing on market’
While Switzerland is no longer part of the European Single Market, due to the lapsed MRA, it still references, and generally adheres to, to the EU MDR/IVDR.
However, there is a significant difference of interpretation regarding ‘placing on the market’.
Unlike in Europe and the UK, where the device is considered placed on the market once transfer of ownership occurs between the manufacturer and the local importer, Switzerland does not consider the device placed onto the market until the Swiss Importer further transfers the device into the Swiss market.
Swissmedic provides a helpful image to clarify the point of ‘placing on the market’.
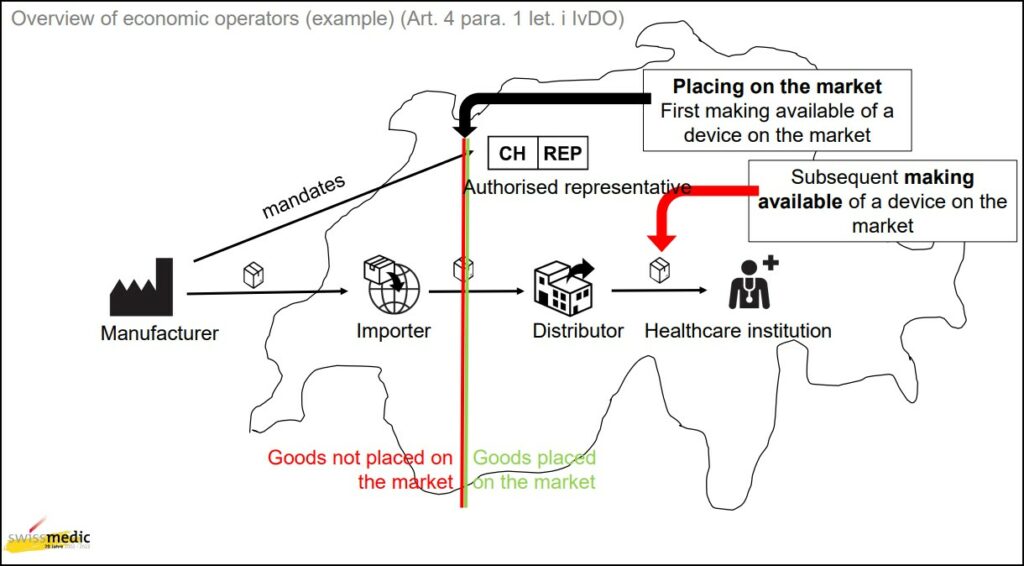
‘Placing on the market’ occurs once the Swiss Importer further transfers the device within Switzerland. It does not occur when the foreign manufacturer transfers ownership of the device to the importer, like it does in the EU and UK.
Switzerland: Example Scenarios
Per Swissmedic’s FAQ titled Stock Items:
Clarification on devices in the manufacturer’s warehouse:
The relevant requirements for devices must be fulfilled at the time of placing on the market (definition according to Art. 4 para. 1 let. b MedDO). Devices in the manufacturer’s warehouse are not yet placed on the market.
Consequence: “Stock sell-off”, e.g. after expiry or withdrawal of the designated body’s certificate, is not possible.
Clarification on devices in the manufacturer’s warehouse:
Devices that were imported or already in the distributor’s warehouse before the new regulation entered into force (26 May 2021) are considered to be placed on the market in the European context according to the provisions of the old legislative and can continue to be made available on the market (Art. 101 para. 3 MedDO; devices compliant with the old legislation may be made available until 26 May 2025 at the latest). The importer or CH-authorised representative does not need to be stated retrospectively.
Devices imported / in the warehouse after the new regulation entered into force (26 May 2021): Transitional provisions apply to the indication of the CH-authorised representative and the CH-importer (for more information see Chapter 6 of the Information sheet, Obligations Economic Operators).
Upon expiry of the transitional provisions applicable to the relevant devices, the importer may place these on the market only if the labelling requirements are satisfied, even if the device had been imported before the expiry of the deadlines.
Take note of the following, related to devices imported after 26 May 2021:
“the importer may place these on the market only if the labelling requirements are satisfied, even if the device had been imported before the expiry of the deadlines.”
Meaning, if a manufacturer ships product to their Swiss Importer (after the MDR/IVDR dates of application), it is not yet considered placed on the market because the ownership of the devices have not yet been transferred from the Swiss Importer to a Swiss distributor, health facility or end user. Even if the manufacturer has sold the product to the Swiss Importer, and in the manufacturer’s mind has ‘transferred the ownership’ from themselves to the Importer, it is not yet considered placed onto the market by Swissmedic’s definition.
Therefore, that stock warehoused by the Swiss Importer must reflect the Swiss Authorized Representative information by the deadlines (vary by classification and between legacy and MDR/IVDR CE marked devices) before the Swiss Importer further moves the goods. If the product was shipped to the Swiss Importer without this information applied, then the Swiss Importer must ensure that the Swiss Authorized Representative information is included by the deadlines, before they place the products onto the market.
For more information on the Swiss Authorized Representative deadlines, please read: Switzerland Authorized Representative