GMDN, EMDN and CND: What is the difference?

Switzerland is a Third Country for medical devices
May 27, 2021
UK: Mid-risk Device Registration Deadline Approaching
August 2, 2021Page Last Updated: 6 February 2024
- What are EMDN codes?
- What do CND codes have to do with EMDN?
- EMDN codes vs GMDN codes
- Can I use my GMDN code to find my EMDN code?
- Are EMDN/GMDN codes required on labeling?
- How are GMDN codes structured?
- How are EMDN codes structured?
- How do I know which EMDN code to select?
- How do I find GMDN codes?
- How do I find EMDN codes?
- How do I request a new EMDN code?
- How do I find CND codes?
- Which is required in the EU, UK, Switzerland?
What are EMDN codes?
EMDN stands for European Medical Device Nomenclature. The EU Commission developed EMDN codes as a way for industry to easily group and identify similar types of products being marketed in Europe.
Per the MDR/IVDR, medical devices and IVDs must be registered in EUDAMED (the European medical device database). And documenting the EMDN code is required in the EUDAMED UDI/Device Registration details.
Why is this important?
The World Health Organization states “there are an estimated 2 million different kinds of medical devices on the world market, categorized into more than 7000 generic devices groups.”
Consider the volume of medical device registrations submitted, renewed and modified globally each day. As well, consider the number of incidents reported, field safety corrective actions (including recalls) initiated each day. This volume makes it critical for international regulators and hospitals to better categorize medical devices.
What do CND codes have to do with EMDN?
Since the EU Commission had to develop a new, free-of-charge nomenclature system, this is where the CNDs come into the discussion.
CNDs are the Italian Ministry of Health’s medical device nomenclature: ‘Classificazione Nazionale Dispositivi medici’. The EU Commission based its new EMDN system on CNDs, which were already in use by the Italian, Greek and Portuguese national competent authorities.
EMDN codes vs GMDN codes
Why did Europe create EMDN codes? Why not use the already widely adopted GMDN code system?
For many years, Global Medical Device Nomenclature (GMDN) codes were the de facto system for categorizing medical devices in Europe. The GMDN database is extensive and recognized by many international regulators, including the US FDA and the Australian TGA. Further, GMDN codes were recognized by European Competent Authorities for well over a decade.
So, if use of the GMDN system was already prevalant in Europe, and the MDR/IVDR required a nomenclature system, why isn’t Europe using GMDN codes?
The reason is monetary. The MDR/IVDR states that the nomenclature system should be free of charge.
While the GMDN Agency does have a Basic membership level available for free, higher levels of membership require payment of an annual fee. The fee varies depending on the size and type of organization. Further, the GMDN Agency prioritizes requests made by paid accounts over requests made by free accounts.
As a result, the Commission decided to develop its own European Medical Device Nomenclature (EMDN) system instead.
“To facilitate the functioning of the European database on medical devices (‘EUDAMED’) as referred to in Article 33, the Commission shall ensure that an internationally recognised medical devices nomenclature is available free of charge to manufacturers and other natural or legal persons required by this Regulation to use that nomenclature.” -MDR Art. 26 & IVDR Art. 23
Can I use my GMDN code to find my EMDN code?
GMDN and EMDN codes are somewhat mapped. However, this mapping is currently only available for manufacturers with a GMDN Agency paid-account. Further, the GMDN Agency caveats that:
“EMDN terms are drafted more widely than GMDN Terms, so one EMDN term is likely to cover numerous types of medical devices, and products falling within different GMDN Terms may be covered by the same EMDN term. The GMDN Agency has no responsibility or liability to you for use of any EMDN term that is identified by this service – it remains your responsibility to ensure you use the most appropriate EMDN term for the relevant medical device.”
If you are eligible, you can try the GMDN Agency’s ‘EMDN Service‘.
The EU Commission has stated that they also intend to have a GMDN to EMDN mapping system. However, it is dependent upon the cooperation of the GMDN Agency:
“To the extent possible, the Commission will map the EMDN to the Global Medical Device Nomenclature (GMDN). This task has been undertaken with the hopes of possibly facilitating EMDN code search by operators currently using GMDN. The correspondence between the nomenclatures is intended to be visible to operators and incorporated in the future database in the form of a searching tool. Therefore, and in cooperation with GMDN, the mapping exercise is currently ongoing.
The level of quality and reliability of this mapping is dependent on the commitment of all relevant parties to work together in mapping and validating the results.”
The GMDN Agency released a paper on the mapping of GMDN codes to EMDN codes. The analysis concluded it was not feasible to easily map between the two nomenclatures.
“Our work shows that a direct term-to-term mapping of the GMDN Terms to the EMDN terminal terms is not fit for grouping medical devices with similar features between different jurisdictions (e.g., between UK or USA, and EU). The other/various named EMDN terminal terms that were predominantly used are not suitable for a direct term-to-term correspondence, because they are broad concepts grouping devices with potentially different features (Figure 2).
In our opinion, this is not an effective approach to the global harmonization of medical devices.”
Of course, one must keep in mind that their conclusion that EMDN codes (free of charge) hinder the “meaningful exchange of information“, benefits the GMDN Agency (pay for optimal service).
Are EMDN/GMDN codes required on labeling?
No. EMDN/GMDN codes are not required to be identified on the product labeling or instructions for use in Europe, the UK or Switzerland.
That said, EMDN/GMDN is required as part of the Unique Device Identification (UDI) system. As a result, the EMDN/GMDN will be contained within the UDI-DI information. The UDI carrier (e.g., barcode) is required on the device labeling, although a transition period has been granted for when the UDI carrier must appear on the labeling. Therefore, in roundabout way, the EMDN/GMDN will be on the labeling, but embedded as part of the UDI-DI.
MDR, Art. 123(f):
- For implantable devices and for class III devices Article 27(4) shall apply from 26 May 2021
- For class IIa and class IIb devices Article 27(4) shall apply from 26 May 2023
- For class I devices Article 27(4) shall apply from 26 May 2025
MDR, Art. 123(g):
with regard to reusable devices that are required to bear the UDI carrier on the device itself, Article 27(4) shall apply to:
- implantable devices and class III devices from 26 May 2023
- class IIa and class IIb devices from 26 May 2025
- class I devices from 26 May 2027
IVDR, Art. 113(e)
- For class D devices, Article 24(4) shall apply from 26 May 2023
- For class B and class C devices Article 24(4) shall apply from 26 May 2025
- For class A devices Article 24(4) shall apply from 26 May 2027
How are GMDN codes structured?
GMDNs have a five-digit numerical code, a Term Name and a Definition.
Below is an example provided by the GMDN Agency on their website:
| GMDN Term Name: | Scalpel, single use |
| GMDN code: | 47569 |
| GMDN Definition: | A sterile, hand-held, manual surgical instrument constructed as a one-piece handle and scalpel blade (not an exchangeable component) used by the operator to manually cut or dissect tissue. The blade is typically made of high-grade stainless-steel alloy or carbon steel and the handle is often made of plastic. This is a single-use device. |
How are EMDN codes structured?
EMDN codes have up to 13 characters, plus an Official Term in Italian and a Draft Term in English.
The structure is made up of: Category + Group + Type + Type(s).
There are up to 7 “levels” and 5 “types”, which each “type” becoming more and more specific to the product.
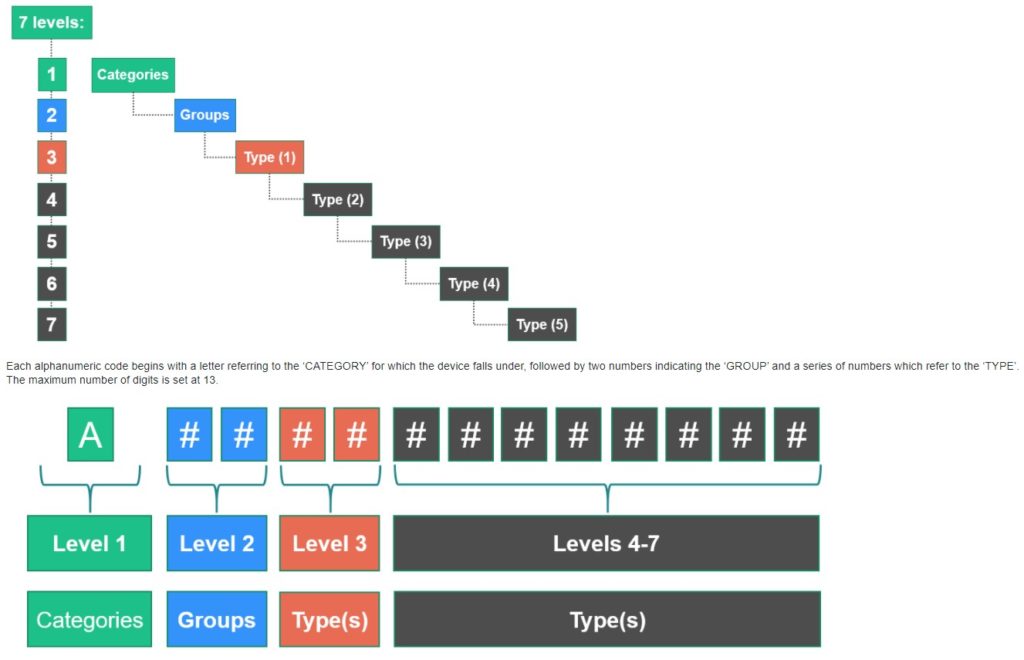
How do I know which EMDN code to select?
There may be a difference between:
- the EMDN you assign to each device and use for EUDAMED registration, and
- the EMDN you assign to the ‘generic device group’ in a technical documentation file
EUDAMED Registration & Each Device
EMDN codes should be assigned at the most granular term available, i.e., the lowest level in the tree.
For example, let us say you manufacture an absorbable synthetic suture:
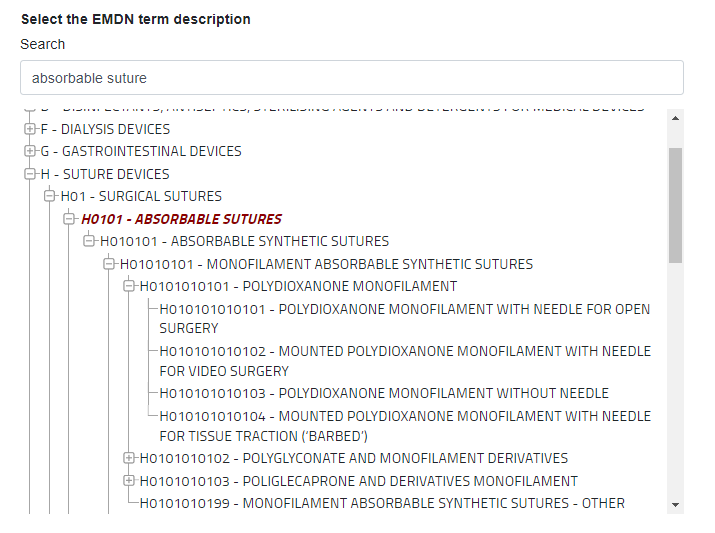
The breakdown is:
- Level 1 (Category) = H – Suture devices
- Level 2 (Group) = H01 – Surgical sutures
- Level 3 (Type 1) = H0101 – Absorbable sutures
- Level 4 (Type 2) = H010101 – Absorbable synthetic sutures
- Level 5 (Type 3) = H01010101 – Monofilament absorbable synthetic sutures
- Level 6 (Type 4) = H0101010101 – Polydioxanone monofilament
- Level 7 (Type 5) = H010101010101 – Polydioxanone monofilament with needle for open surgery
- Level 7 (Type 5) = H010101010102 – Mounted polydioxanone monofilament with needle for video surgery
- Level 7 (Type 5) = H010101010103 – Polydioxanone monofilament without needle
- Level 7 (Type 5) = H010101010104 – Mounted polydioxanone monofilament with needle for tissue traction (‘barbed’)
Using the above device as an example, one of the four “Level 7 (Type 5)” codes would be assigned to each device, as applicable. And it would also be used in the EUDAMED registration for each device, as applicable.
MDR/IVDR Technical Documentation File Purposes
According to EU guidance document MDCG 2019-13:
“generic device group is to be understood, in respect to the MDR as the 4th level of the EMDN and in respect to the IVDR as the 3rd level of the EMDN in combination with the most appropriate IVP code.”
However, note the caveat that:
“If the notified body considers that for a particular device level 4 for the MDR/level 3 for the IVDR is not sufficiently specific to define a generic device group, it can use the next lower level if available.”
Therefore, using the above device as an example, the applicable 4th level EMDN code would be ‘H010101 – Absorbable synthetic sutures’.
How do I find GMDN codes?
Visit our page: How to Find GMDN Codes for Free
How do I find EMDN codes?
The EMDN database is located: HERE
Unlike the GMDN database, you may download the full Excel list of EMDN codes: HERE
However, note that that EMDN codes may be modified and/or added as needed, making a downloaded Excel or PDF list outdated over time.
Your EMDN code will be linked to the Unique Device Identifier – Device Identifier (UDI-DI) as part of the EUDAMED device registration process. As well, manufacturers should identify the EMDN code in its technical documentation file and procedures as applicable.
How do I request a new EMDN code?
If you cannot find an applicable EMDN code, you may request a new code be created.
Process
- You must have an EU log-in, to request a new code. If you have registered in EUDAMED, you will already have one. If you have not, create one: HERE
- It is a simple process, requiring your name, email address, and creation of a password.
- To request a new EMDN code, click: HERE
- If you are not signed into your EU log-in, the page will prompt you to do so. You do not need to leave the page. Simply click the link in the prompt and sign-in.
- Once logged in, a Request Form will appear.
- Enter the details requested, along with any supporting information as needed.
- Submit.
Once submitted, it will be assessed by the EMDN Technical Team and MDCG Nomenclature Working Group.
You may also use the above application to request an EMDN code amendment.
Timing
EMDN codes are only assessed once per year and the cutoff date is January 31 of each year. For example:
- You submit your EMDN code request in January 2024. Your request will be reviewed, and a decision made by December 2024.
- You submit your EMDN code request in March 2024. Your request will be reviewed, and a decision made by December 2025.
What if I need an EMDN code faster?
There is a process to submit ad hoc requests; however, it is only available to Competent Authorities and Notified Bodies. If you have an urgent need for a new code, you may need to involve support from your Notified Body.
How do I find CND codes?
CND codes can be found on the Italian Competent Authority’s website: HERE
The list of CND codes is available in excel and PDF and is available for download.
Which is required in the EU, UK, Switzerland?
The following nomenclature systems are required for each market:
European Union
EMDN codes are required when completing the device registration in EUDAMED.
EUDAMED was originally scheduled to go live in May 2020. However, the EU Commission postponed EUDAMED. In the meantime, various modules have been released for voluntary use, including the device registration module.
For more information on timing, and the process to voluntarily register early, please read: EUDAMED Basics
For information on the current registration requirements until EUDAMED is mandatory, please read: EU Registration Requirements
Switzerland
EMDN codes are required when completing the device registration in swissdamed.
The Mutual Recognition Agreement (MRA) between Switzerland and the EU has lapsed for medical devices. As a result, Swissmedic no longer has access to EUDAMED, other than the publicly available version.
The Swiss Federal Council is still hopeful to eventually resolve the MRA issue. In the meantime, Switzerland is in the process of developing its own EUDAMED-like registration database called ‘swissdamed’.
For more information, please read: Swiss Medical Device Registration Requirements
United Kingdom
GMDN codes are required when completing the device registration in the MHRA’s system.
For more information, please read: UK MHRA Medical Device Registration Requirements


