EUDAMED Actor Registration FAQs

Switzerland to implement IVDR
March 4, 2022
Swissmedic Updates Registration Guidance & Announces New “Swiss EUDAMED”
March 15, 2022Page Last Updated: 23 January 2024
- How do I fit into the EUDAMED system?
- What exactly is a EUDAMED ‘Actor’?
- Are all Economic Operators also EUDAMED Actors?
- I am an Economic Operator and a EUDAMED Actor. What do I need to do?
- I perform multiple Economic Operator roles. How do I register correctly?
- What are my ongoing responsibilities as an Actor?
- What is the deadline to register in EUDAMED?
- What are the current registration requirements?
The EUDAMED Actor Registration module launched on 1 December 2020. This was the first of the six EUDAMED modules to be made available. Find out below who needs to register as a EUDAMED Actor.
How do I fit into the EUDAMED system?
EUDAMED’s goal is to “provide a living picture of the lifecycle of medical devices that are made available in the European Union (EU)”. To accomplish this, the key organizations involved with the product’s lifecycle need to be identified and linked. Within EUDAMED, those organizations are referred to as ‘Actors’.
Please find here links to the EUDAMED websites: Public Website, Registration Website
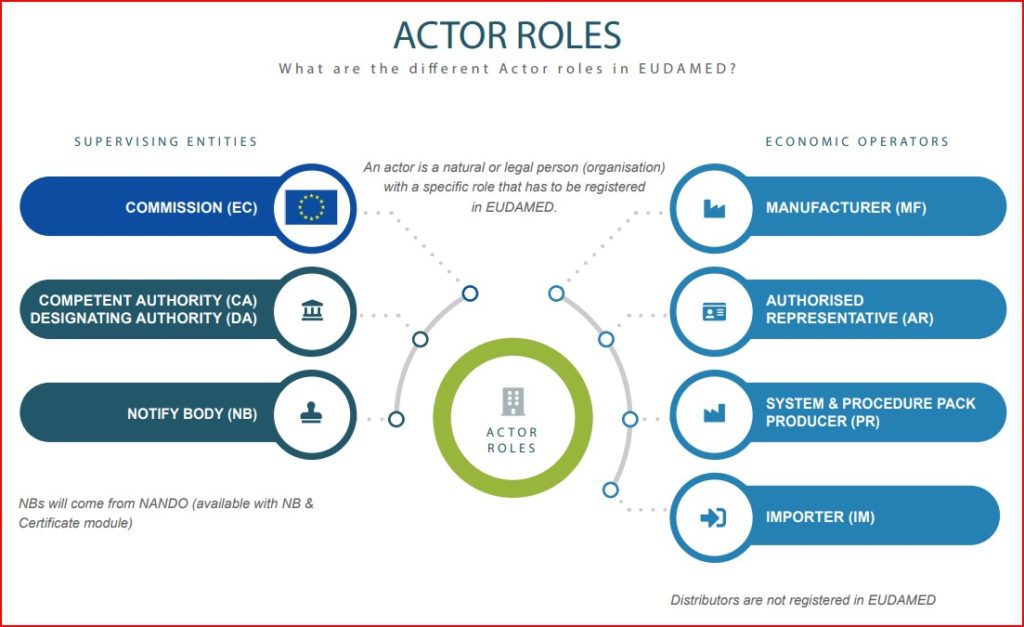
What exactly is a EUDAMED ‘Actor’?
A EUDAMED Actor is any natural or legal person (i.e., organization) that must be registered in EUDAMED. Actors are divided into two groups:
- Supervising Entities and
- Economic Operators.
Supervising Entities are those responsible for medical device oversight, including CE Marking certification. Specifically, these are: the European Commission, Competent Authorities, Designating Bodies, and Notified Bodies.
‘Economic Operators’ are the entities involved in the supply chain. Specifically, Economic Operators are defined in the MDR/IVDR as: Manufacturers, Authorized Representatives, Importers, Distributors and System/Procedure Pack Producers.
Are all Economic Operators also EUDAMED Actors?
Not all MDR/IVDR Economic Operators are EUDAMED Actors. The following are Economic Operator Actors that require registration in EUDAMED: all manufacturers, EU authorized representatives, EU importers and all System/Procedure Pack Producers.
While distributors are economic operators, they do not register in EUDAMED. If a distributor acts as an EU importer for some devices, then they become an Actor. In this case, they must register an importer in EUDAMED – but only for those specific devices. They would not link themselves to any manufacturers / devices for which they are only the distributor.
I am an Economic Operator and a EUDAMED Actor. What do I need to do?
Manufacturers, System/Procedure Pack Producers, EU Authorized Representatives and EU Importers must all register in EUDAMED’s Actor Registration Module and obtain a Single Registration Number (SRN). The SRN is your company’s unique identification code and links your organization, role and devices. The SRN is a key component of EUDAMED’s traceability functionality.
Next, Manufacturers and System/Procedure Pack Producers must register their devices in EUDAMED’s UDI/Device Registration Module. Once the device registration has been completed, Importers will link themselves to the devices that they import into Europe.
I perform multiple Economic Operator roles. How do I register correctly?
A single SRN cannot be used for multiple roles. Each SRN is specific to a single Economic Operator role. Meaning, if your organization is a manufacturer and is also a System/Procedure Pack Producer, you need to register in EUDAMED twice and obtain two SRNs. This is true even if the company name, address and Person Responsible for Regulatory Compliance are all exactly the same. The Economic Operator role is different, and that is the key point.
What are my ongoing responsibilities as an Actor?
In addition to registering as an Actor, obtaining your SRN and registering devices, ongoing EUDAMED obligations include:
- Enter clinical investigation and performance study data
- Upload Summary of Safety and Clinical Performance (when applicable)
- Enter incident reports
- Upload Field Safety Notices
- Ensure registration data remains current and complete
The responsibility of which Actor enters this data (e.g., Competent Authorities, Manufacturers) may vary. As well, some EUDAMED functions are not yet available, such as the Clinical Investigations and Performance Studies, Vigilance and Post-market Surveillance, and Market Surveillance Modules.
What is the deadline to register in EUDAMED?
The EUDAMED is currently voluntary; the mandatory compliance date has been postponed. For the most current understanding of EUDAMED’s deadlines, please read: EU Commission Proposes EUDAMED Gradual Rollout
Regardless, it is a good idea to get started now and avoid the rush at the deadline. The reasons are:
- An extremely large number of organizations must register, and all SRN registrations must be reviewed by a European National Competent Authority before it is issued.
- If you are a manufacturer located outside of the EU, your Authorized Representative must also review the SRN registration application, in addition to the Competent Authority. This potentially creates a bottleneck if you leave it until the deadline.
- Importers must link to the devices they import. If you are delayed on your EUDAMED registration, then they will also be delayed on their registration compliance.
- Some competent authorities, like the Irish HPRA, are already requiring EUDAMED registration.
What are the current registration requirements?
Until EUDAMED is mandatory, the registration requirements under the MDD/AIMDD/IVDD continue to apply.
For an overview of which devices require registration, and where, please read: EU Medical Device & IVD Registration Requirements


